Triple speed

Scale-up quickly

Adapt throughput to your needs

Easy integration

Inhibitor-free nucleic acids

Reduced consumable costs
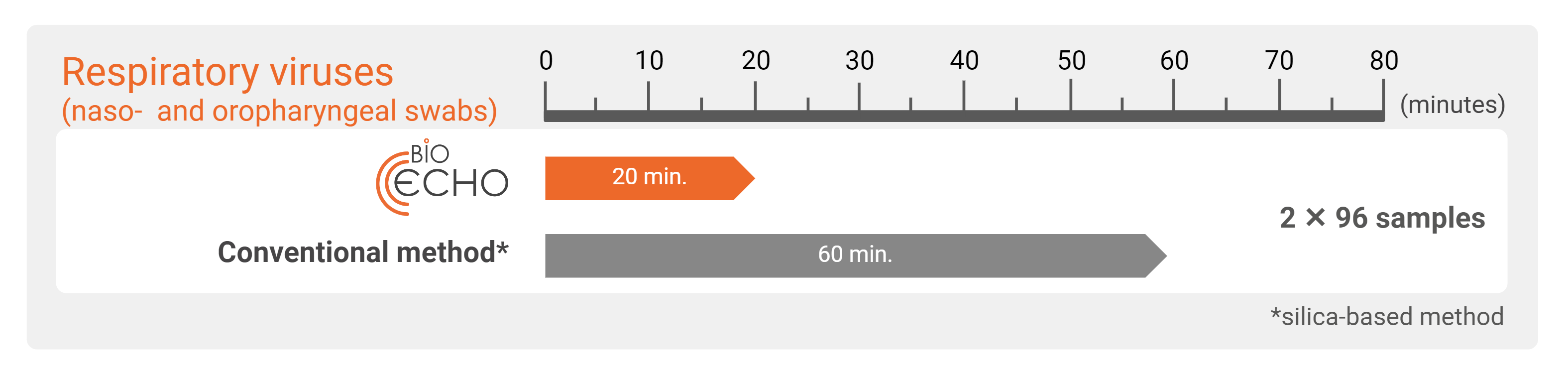
With the EchoLUTION technology, no incubation is needed for viral lysis. After a centrifugation step to prepare the column or plate, the purification is accomplished in a single one-minute centrifugation. Compared to silica-based methods using the bind–wash–elute principle that include several centrifugation steps, prolonging the workflow drastically.
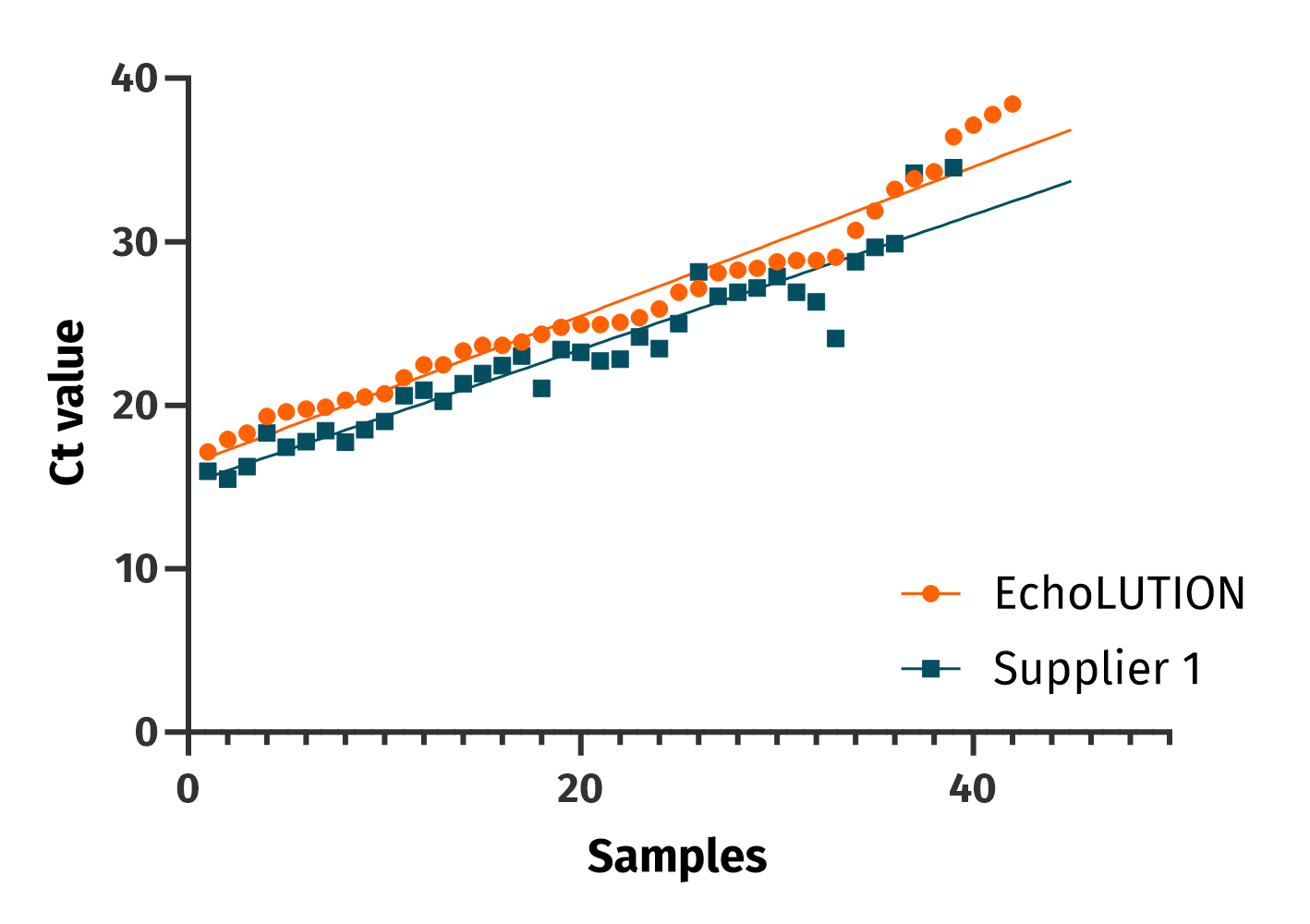
Comparable performance
The clinical performance between EchoLUTION and a magnetic beads-based kit for the extraction of SARS-CoV-2 RNA is similar. The BioEcho viral kit (orange) and the magnetic beads kit (blue) were used to analyze 62 patients’ samples for the detection of the RdRP gene.

Moritz Hass,
Lab Manager, MVZ Sonnen-Gesundheitszentrum SOGZ, Germany

“Tourism is such an important industry for Cyprus and our laboratories must be as efficient as possible to deal with the huge numbers of passengers that visit our island every year. Thanks to the innovative extraction system of BioEcho, we have been able to eliminate bottlenecks at peak times and increase the overall efficiency of our COVID-19 PCR testing operations in a cost-effective manner without compromising the quality of our results”.
Dr. Christos Shammas,
Director of Bioanalysis, Limassol, Cyprus

“The automated RNA extraction workflow allowed us to process the large volumes of samples we received from hospitals and others much faster. The most important thing in fighting the Corona pandemic, especially at the beginning, was not to be able to send a positive result days later, but within 24 hours.”
Dr. Thomas Fenner,
Managing Director, Fenner laboratory, Germany


Talk to a nucleic acid expert!
Do you have questions about viral DNA and RNA extraction?