Get results faster

Simplify workflows significantly

Adapt throughput to your needs

Inhibitor-free nucleic acids

Reduced consumable costs
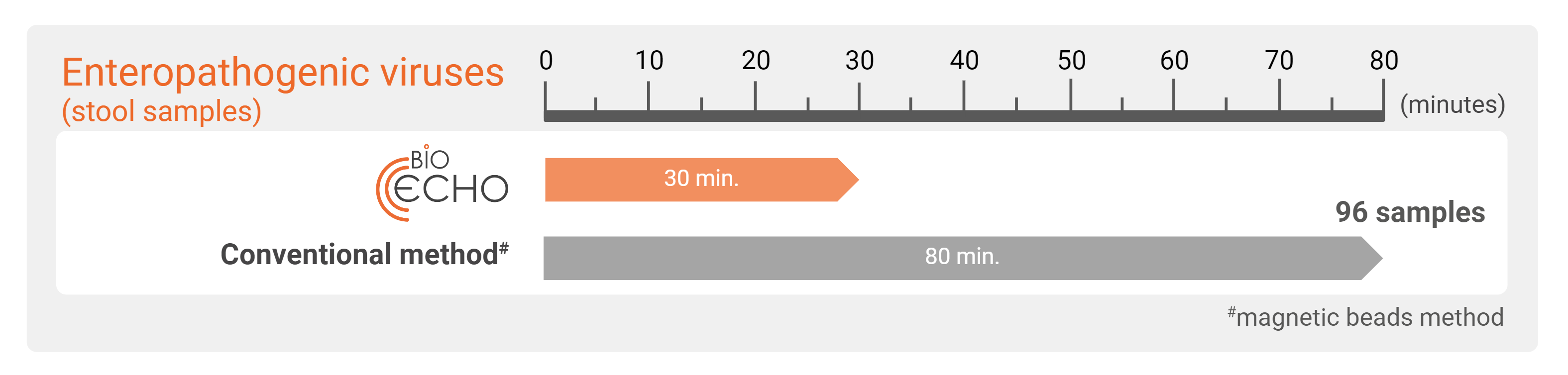
With the EchoLUTION technology, only 10 minutes incubation is needed for enteropathogenic viral lysis. After a centrifugation step to prepare the column or plate, the purification is accomplished in a single one-minute centrifugation. Compared to methods based on the bind–wash–elute principle including several centrifugation steps, the EchoLUTION technology is up to three times faster.
Robust qPCR efficiency
To evaluate our EchoLUTION technology, positive stool samples from patients were stored frozen and used to determine qPCR efficiency. The samples were validated for clinical diagnosis of adenovirus and rotavirus. Results confirmed that the EchoLUTION technology exhibited suitable efficiency, confirming that no inhibitors are present in the sample after nucleic acid extraction.
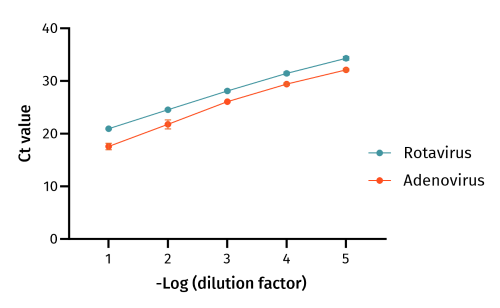
(RT)-qPCR of a dilution series from nucleic acids extracted using the EchoLUTION Viral RNA/DNA Kit. (RT)-qPCR quantification cycle (Ct) values are shown on a logarithmic scale. N = 8 replicates for each dilution and virus type.

The qPCR efficiency (%) has been calculated based on the slope of the log linear regression. Samples isolated with the EchoLUTION kit exhibit a reliable qPCR efficiency. Optimal efficiency lies between 90 – 110 %.

Dr. rer. medic. Christoph Schönfels,
Head of PCR Department, Medizinische Labor Ostsachsen MVZ GbR, Görlitz, Germany


Talk to a nucleic acid expert!
Do you have questions about viral DNA and RNA extraction?